Phase 3 trial demonstrates practice-changing survival benefits in muscle-invasive disease
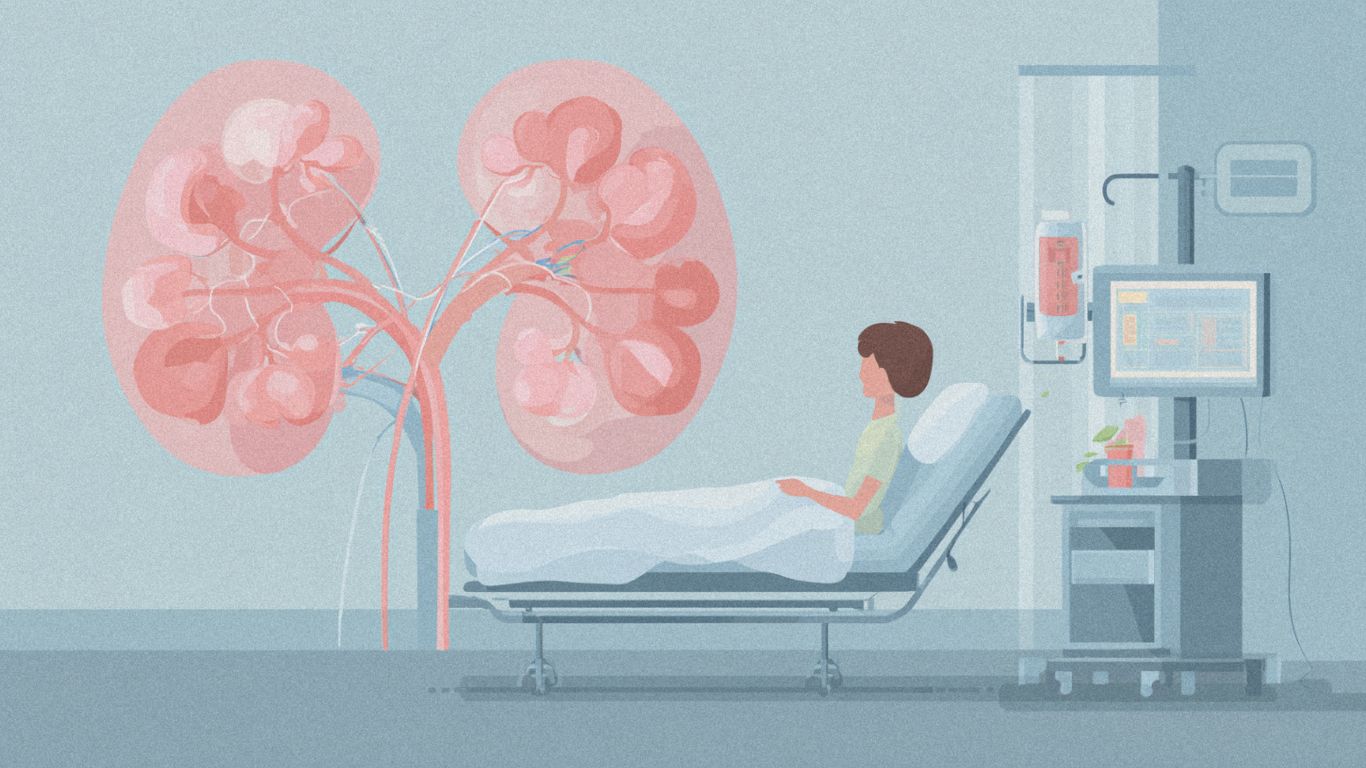
Pfizer announced highly positive interim results from a pivotal Phase 3 trial combining its antibody-drug conjugate Padcev (enfortumab vedotin) with Merck’s Keytruda (pembrolizumab) in muscle-invasive bladder cancer, marking a potential paradigm shift in treatment approach.
The combination, administered both before and after surgery (neoadjuvant/adjuvant therapy), significantly improved both event-free survival and overall survival compared to surgery alone in patients with muscle-invasive bladder cancer. The results, reported August 12, underscore what researchers are calling the “practice-changing potential” of this dual-agent regimen in earlier-stage disease.
Clinical Impact: This represents the first successful perioperative therapy to demonstrate survival benefits in muscle-invasive bladder cancer, addressing a critical unmet need in urology. The combination builds on already-proven efficacy in metastatic settings, where Padcev plus Keytruda has shown substantial survival advantages.
The success validates Pfizer’s $43 billion acquisition of Seagen, which brought the antibody-drug conjugate platform into its portfolio. Padcev delivers a cytotoxic payload directly to cancer cells expressing Nectin-4, while Keytruda unleashes the immune system’s natural anti-tumor response.
Regulatory Timeline: Pfizer plans immediate discussions with global regulators to seek expanded approvals based on these data. Current approvals for the combination cover advanced/metastatic urothelial cancer, but this study positions it for earlier-stage, curative-intent treatment.
The results strengthen Pfizer’s oncology franchise as the company faces patent cliffs on other key products, providing a significant growth driver in the high-value cancer therapeutics market.